Introduction
Acute myeloid leukemia (AML) is highly heterogeneous disease with broad spectrum of mechanisms involved in pathogenesis. The unique microenvironment of hematopoietic niche (HN) consists of several proteins with corresponding receptors on normal and leukemic cells. The interactions within HN include ligand-receptor pairs as SDF-1/CXCR4; OPN/CD44; Jagged-1/Notch-1; ANG-1/Tie-2 and Slit2/Robo4. MicroRNAs (miRNAs) are small noncoding RNA molecules that post-transcriptionally modulate gene expression by binding to targeted mRNAs. The dysregulation of ligand-receptor interactions by miRNAs could disrupt the niche's equilibrium and may contribute to the establishment of a leukemic burden and progression of AML.
In our study we aimed to explore the clinical impact of miRNAs profile in the context of the mRNA genes expression involved in ligand-receptors interactions in the bone marrow of newly diagnosed AML patients (pts).
Methods
The expression of 12 mature miRNAs: miR-15a-5p, miR-34a-5p, miR-125b-5p, miR-139-5p, miR-146-5p, miR-181a-5p, miR-199b-5p, miR-204-5p, miR-218-5p, miR-299-5p, miR-424-3p, miR-452-5p was determined in duplicates in bone marrow samples of de novo AML pts using real-time PCR. The miRNAs expression was normalized using the mean expression of all miRNAs in a given sample.
The expression assessment of 10 following genes: CXLC12 (coding SDF-1 ), CXCR4, NOTCH1, JAG1 (coding Jagged-1), SPP1 (coding osteopontin) , CD44, SLIT2, ROBO4, ANGPT1 (coding angiopoietin-1) , TEK (coding Tie-2 receptor) was performed in the bone marrow at the time of AML diagnosis. The gene expression was determined in duplicates by real-time PCR using the QuantStudio 7 (Applied Biosystems-ThermoFisher Scientific). Gene expression was normalized using selected reference genes ( MRPL19 and PPIA).
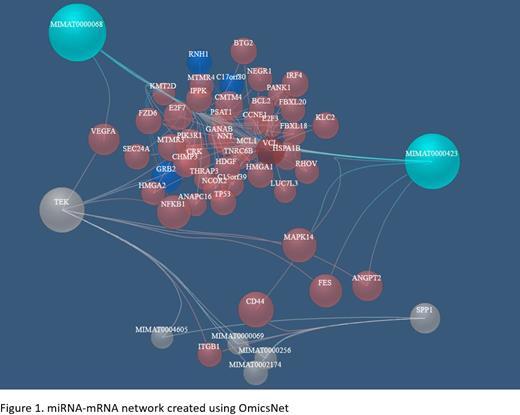
Differential expression analysis was conducted using a heteroscedastic t-test, volcano plots were used to visualize results. Using significantly deregulated miRNAs and genes in high-risk AML, OmicsNet (https://www.omicsnet.ca) was used to create miRNA-mRNA biological network. For network creation, protein-protein interactions (STRING) and miRNA-mRNA (TarBase) databases were used. Further, a pathway enrichment analysis was performed using Reactome database.
Results
The study group consisted of 28 (16 women and 12 men) newly diagnosed AML adults with the mean age 57.7 ± 12.8 years. Among them, 14.3% pts had a low-risk, 39.3.% intermediate-risk and 39.3% high-risk AML according to European LeukemiaNet ( ELN) 2022 risk stratification. In 2 pts molecular data was not available. The most common subtype was AML not otherwise specified (11 pts, 39.3%), followed by AML with myelodysplasia-related cytogenetic abnormality (6 pts, 21.4%), AML with myelodysplasia-related gene mutation (5 pts, 17.9%), and AML with recurrent genetic abnormality (4 pts, 14.3%). At diagnosis the median white blood cell count was 26.7 × 10 9/L (interquartile range, IQR: 3.0 - 73.1 × 10 9/L), the median bone marrow blasts was 63.0% (IQR: 41.0- 88.3) and the median lactate dehydrogenase (LDH) level was 532.5 U/L (IQR: 284.0-986.5).
The majority of pts (86%) received intensive chemotherapy according to Polish Adult Leukemia Group (PALG) protocols. In FLT3 mutated pts standard induction/consolidation therapy was enriched with midostaurin. Low intensity treatment consisted of azacitidine (1 pts), low dose Ara-C (LDAra-C, 2 pts) and LDAra-C + cladribine (1 pts).
Patients with high-risk AML had higher expression of miR-125b-5p (fold change- FC=2.3, p=0.017) and lower expression of miR-15a-5p (FC=0.6, p=0.043) as compared to low- and intermediate-risk pts. Similarly, two genes were significantly upregulated in ELN high-risk group - TEK (FC=4.1, p=0.017) and SPP1 (FC=6.7, p=0.041).
OmicsNet was used to construct miRNA-gene interaction networks between miR-125b-5p, miR-15a-5p, TEK, SPP1 and other miRNAs target genes. In final multi-omic network, 6 miRNAs and 50 mRNA/proteins were included (Figure 1). We found that the pathways of PDGF, NGF, FGFR, PI3K, IGF1R and EGFR signaling are significantly enriched in high-risk AML patients.
Conclusions
Our preliminary results indicate the signaling pathways disturbed in high-risk AML patients. The miR-125b-5p, miR-15a-5p as well as the TEK and SPP1 gene expression may possibly act as biomarkers in patients with de novo AML.
Disclosures
Czemerska:Celgene/BMS: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Sandoz: Honoraria. Strzalka:Celgene/BMS: Honoraria. Krawiec:Celgene/BMS: Honoraria. Pluta:Celgene/BMS: Honoraria; Astellas: Honoraria; Jazz Pharmaceuticals (Swixx): Honoraria, Research Funding; Abbvie: Honoraria; Pfizer: Honoraria. Wierzbowska:Astellas: Consultancy, Honoraria; Celgene/BMS: Honoraria; Pfizer: Honoraria; Servier: Honoraria; JazzPharmaceuticals/swixx: Honoraria; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal